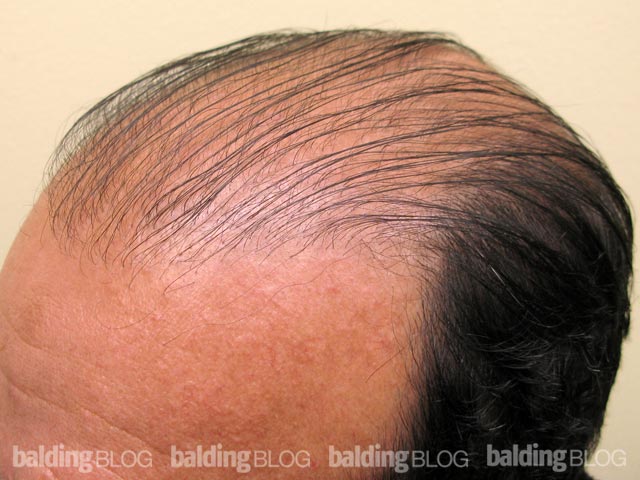
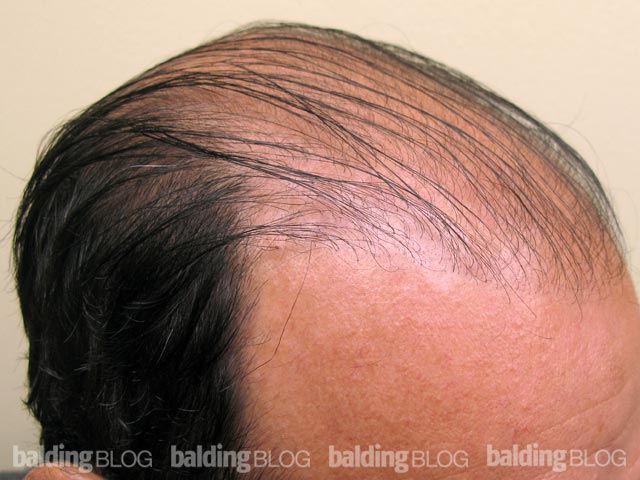
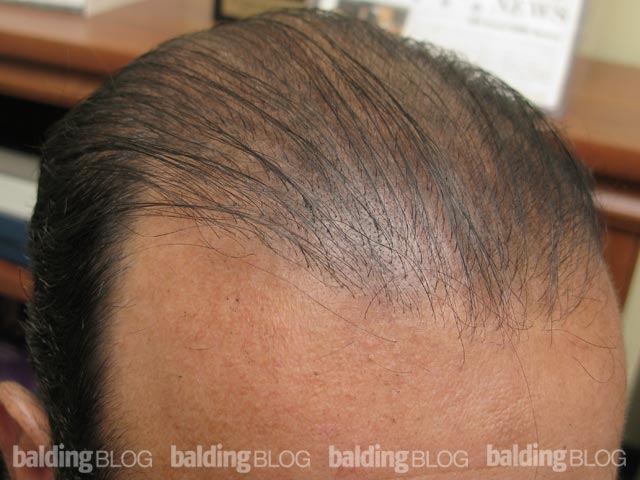
Under what circumstances would you consider prescribing dutasteride? I am a 23 year old male and a current NW 3. I am thinking about asking my doctor about dutasteride, but I would like to know my risks. I have been on propecia for 5 years and I have had great results, but now I am noticing more thinning. I did not have any sides effects from finasteride. Do the results from the Korean study reveal any substantial risks? Is there any indication that Avodart is seeking FDA approval? If someone were to experience sexual side effects can you discontinue and retain sexual performance?
 As far as I’m aware, results from the 6 month clinical trials in Korea have yet to be made public, so I don’t know what risks were revealed. It is a phase 3 study, so I assume that means they want the drug to go to market as a hair loss treatment. The phase 1 and 2 trials were stopped for unknown reasons, though. I don’t have the answers to your other questions, as I was not a part of the study and am not privy to any results.
As far as I’m aware, results from the 6 month clinical trials in Korea have yet to be made public, so I don’t know what risks were revealed. It is a phase 3 study, so I assume that means they want the drug to go to market as a hair loss treatment. The phase 1 and 2 trials were stopped for unknown reasons, though. I don’t have the answers to your other questions, as I was not a part of the study and am not privy to any results.
If it eventually is FDA approved for the treatment of genetic male pattern hair loss, I’d guess that GlaxoSmithKline (the maker) will release the dutasteride under a different brand name from the Avodart name and possibly with a lower dosage, much like Merck did with Proscar/Propecia (both of which are finasteride, but in different dosages). This is all speculation though, and the medication might never be approved as a hair loss treatment. I find it interesting that the phase 1 and 2 trials were stopped and would like to know why the phase 3 trial results are taking so long to be released.
Tags: avodart, dutasteride, hairloss, hair loss, glaxosmithkline, gsk, propecia, proscar, finasteride, fda

 As far as I’m aware, results from the
As far as I’m aware, results from the 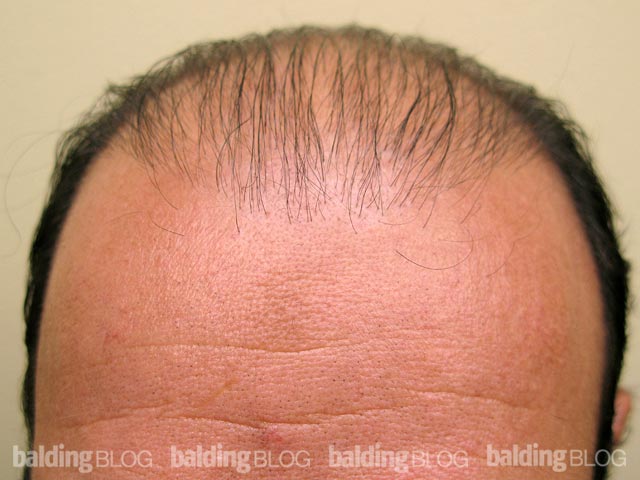


 For those that don’t know what this show is, check out this post:
For those that don’t know what this show is, check out this post:  This rebate is a great way to cut the costs of Propecia, particularly if you’ve wanted to give the medication a try and the price has been prohibitive… or if you just like to save money (and who doesn’t?).
This rebate is a great way to cut the costs of Propecia, particularly if you’ve wanted to give the medication a try and the price has been prohibitive… or if you just like to save money (and who doesn’t?).