The frontal line is straight and the grafts are put into a line like soldiers so that is not normal. I can’t tell you about the location of the hairline because it does not show the frontal view with the eyebrows lifted high so that the forehead creases show up. I also can’t tell if the frontal hairs are all single hairs (at least 1/8th inch distance from the leading edge should have only single hairs in the front).
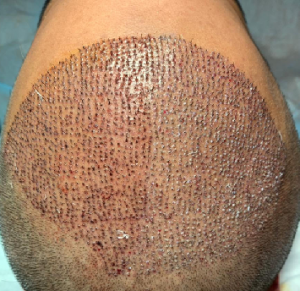
I’ve been on generic finasteride 0.5mg for 6 weeks now and have experienced a week of low libido which went away within the first few weeks. I have not experienced any noticeable amount shedding so far. I’ve been considering upping the dose to 1mg. If I were, will I experience a shed within the next 3 months as if I were only just starting the medication or would it continue alongside the last 6 weeks of 0.5mg? Will there be a noticeable effect? The only reason I am taking 0.5 is for financial reasons, so if it’s best to stay on that then I might do so.
I doubt that you will experience shedding at the 1 mg dose considering that you are on it now. The libido is another story. If you should see an impact on libido on the 1 mg dose, go back to the half dose as it is 80% as effective. A recent study shows that there is far less sexual side effects than the public believes, see here: https://baldingblog.com/new-report-that-finasteride-does-not-cause-sexual-side-effects/
https://www.ncbi.nlm.nih.gov/pubmed/26636418
Int J Clin Pharmacol Ther. 2016 Jan;54(1):19-27. doi: 10.5414/CP202467.
Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia.
Abstract
OBJECTIVE:
The effects on scalp and serum dihydrotestosterone (DHT) of different doses of a novel topical solution of 0.25% finasteride (P-3074), a type 2 5?-reductase, were investigated in men with androgenetic alopecia.
METHODS:
Two randomized, parallel-group studies were conducted. Study I: 18 men received 1 mL (2.275 mg) P-3074, applied to the scalp once a day (o.d.) or twice a day (b.i.d), or 1 mg oral tablet o.d. for 1 week. Study II: 32 men received P-3074 at the dose of 100 (0.2275 mg), 200 (0.455 mg), 300 (0.6285 mg), or 400 (0.91 mg) ?L or the vehicle o.d. for 1 week. Scalp and serum DHT and serum testosterone were evaluated at baseline and treatment end.
RESULTS:
Change from baseline in scalp DHT was -70% for P-3074 o.d. and approx. -50% for P-3074 b.i.d. and the tablet. Serum DHT decreased by 60 – 70%. The doses of 100 and 200 ?L P-3074 resulted in a -47/-52% scalp DHT reduction, similar to the 300 and 400 ?L doses (i.e., -37/-54%). A -5.6% inhibition was observed for the vehicle. Serum DHT was reduced by only -24/-26% with 100 and 200 ?L P-3074 and by -44/-48% with 300 and 400 ?L P-3074. No relevant changes occurred for serum testosterone.
CONCLUSIONS:
The novel finasteride 0.25% solution applied o.d. at the doses of 100 and 200 ?L results in an appropriate inhibition of scalp DHT potentially minimizing the untoward sexual side-effects linked to a systemic DHT reduction.
Why do so many people complain about bumps/skin discoloration after beard transplants? Is this an unavoidable side effect?
Bumps in the skin should not occur if the grafts are trimmed of most of its skin and placed flush with the surface of the recipient area?
Has there been any definitive studies done on which genes have an effect on post-finasteride syndrome (side effects that persist after discontinuation of Fin)? ARTICLE: https://www.smoa.jsexmed.org/article/S2050-1161(16)30075-7/abstract
Very interesting. I just read the full article and I will quote the last of the result analysis and the conclusion of the article. My interpretation suggests that the ability to detect men who might get PFS is not understood by any of the genetic findings of this report; however, there are some influences of symptoms reported by men who take finasteride as summarized below:
” A general limitation of this study work is that some symptoms reported by patients with PFS could not be objectively determined. Furthermore, the retrospective design of our study did not allow a clinical assessment of these men before finasteride use. Future studies are necessary to assess the AR genetic profile and testosterone levels in subjects who developed PFS compared with subjects who did not develop adverse symptoms after using finasteride against AGA. CONCLUSION Causes and predisposing factors responsible for the development of long-term adverse side effects in young men who used low-dose finasteride against AGA remain an enigma. Several symptoms were in common in more than 70% of patients with PFS, but a plethora of other disturbances was reported by a minority of patients, with some clearly related and some not to androgenicity. Our study showed that the length of two trinucleotide repeats in the AR gene contribute to the frequency of some specific symptoms reported by patients with PFS. The (CAG)n and (GGN)n polymorphisms were involved in two specific symptoms (ie, scrotal discomfort and increased skin dryness); for other symptoms, only one of the two polymorphisms was involved, which is likely a reflection of the complex modulation of AR activity.16,40 Our investigation using a precision medicine approach suggested genetic implications in symptoms of patients with PFS. Much more genetic and non-genetic research is necessary to elucidate the pathophysiologic pathways leading to the onset and persistence of adverse effects in former finasteride users.”
Page 6 of 6