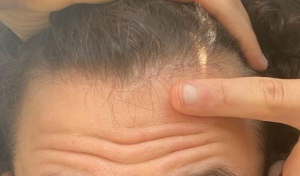
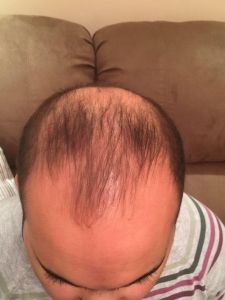
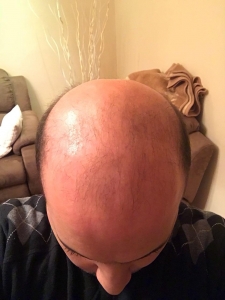
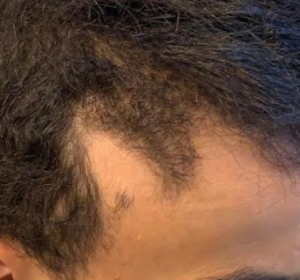
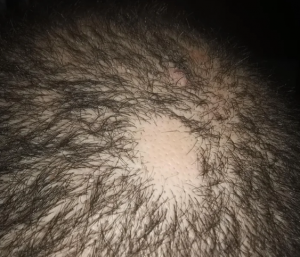
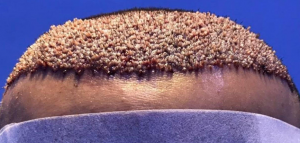
The crown isn’t a styling area, mostly just need the look of fullness over there. It’s common just to get that area micropigmentation. So why not just use some body hair to fill it in? I know there’s a reason why, otherwise it would be done more. But what is it?
Body hair donor sites have problems with them as follows: (1) they grow to a limited length, (2) they only grow about 6-8 months and then fall out, (3) their texture (bulk) is less than most scalp hair so that it takes many more to be equal to a scalp donor hair and (4) half of all of the transplanted hairs are in telogen (sleeping) half of the time which means that for every 10 hairs that are transplanted, only 5 are growing at any one time. In conclusion, it is not practical and very expensive. As for hair elsewhere in the body, beard as a donor source is good but not for the frontal area and only when you run out of good scalp donor hair. Neck hair is not permanent hair so it should never be used as a donor source.
I am a female. My dermatologist recently told me that any kind of hair color should not be used when experiencing hair loss/thinning hair. I’m dealing with thinning hair on my crown and right side of my scalp. I’ve recently started using Rogaine and my derm advised I could use whatever shampoo I wanted, ( I was very surprised at this). I’ve been using semi-perm color on my hair for years and on occasion using perm color. All in the salon ?
Yes, hair coloring impacts the hair on top of your head. Genetic hair loss is below the scalp level so the two are unrelated. Don’t damage your hair with dyes, which can happen below the skin when it is used wrong.
The issue may be: ‘Do you want to travel to Turkey (airports, airplanes, hotels in a city that may have a lot of Virus there)?
HAIR GROWTH DRUG SEEN AS A WONDER FOR UPJOHN
By John Crudele

See the article in its original context from
May 28, 1985, Section D, Page 1Buy Reprints
May 28, 1985, Section D, Page 1Buy Reprints
The Upjohn Company may be several years away from receiving Government approval to sell what could be a revolutionary hair-growth ointment, but thousands of balding men are said to be using a homemade version of the product.
And while the company is still a long way from seeing the full financial impact of its drug, called minoxidil, Upjohn may already be realizing considerable benefits from the cottage industry that has grown up around the compound.
Minoxidil has the approval of the United States Food and Drug Administration, but as a tablet taken to treat hypertension. Years ago, however, Upjohn scientists found that the drug promoted hair growth as a side effect. More recently, doctors have been putting patients on minoxidil not to fight hypertension, but to grow hair.
Dr. Michael Lorin Reed, assistant clinical professor of dermatology at the New York University Medical Center, said he had ”at least several hundred people on the drug right now.” Dr. Reed said there were a ”zillion people prescribing” minoxidil for hair growth.
No Final Verdict on Product
While such use by doctors is legal, the F.D.A. said, people might not get the desired results. Upjohn, meanwhile, has been contending that some sellers of the homemade product may be infringing on company patents.
A question remaining about the drug’s use in combating baldness is whether it will be absorbed through the skin and produce unusually low blood pressure.
While a debate is raging over how effective the drug will be against receding hairlines, Wall Street is unanimous in believing the product will be important for Upjohn.
”Topical minoxidil for male pattern baldness could become one of the largest selling drugs in the world and transform Upjohn into one of the fastest growing major domestic drug companies,” said Ronald M. Nordmann, an analyst at Oppenheimer & Company.
Stock Soared Last Week
The investment community’s excitement over the hair ointment was obvious last week when the mention of minoxidil in a routine report by another Wall Street analyst, Paul Brooke of Morgan Stanley & Company, sent Upjohn’s stock soaring $13.375 a share for the week to a final price of $110.25 on Friday.
Mr. Nordmann believes minoxidil, if approved by the F.D.A. for external use, could generate about $500 million in annual sales for Upjohn and net income of $204 million. Upjohn’s total sales in 1984 were $2.18 billion and net earnings were $173.3 million.
But Upjohn may already be seeing some benefits of minoxidil’s popularity for hair growth. While Upjohn will not disclose sales figures for any of its drugs, Mr. Nordmann estimated that sales of minoxidil tablets, under the brand name Loniten, would grow to about $30 million this year, from only $7 million in 1983. ”The growth is clearly not coming from the hypertension market,” he said.
It was in the early 1970’s that Upjohn, a pharmaceutical company based in Kalamazoo, Mich., noticed that minoxidil tablets were causing hair growth in patients. In 1977 it began investigating whether the drug, when applied externally as a liquid, could arrest the balding process. An Upjohn spokeswoman said the results of the studies were not complete, but she added that the company expected to file for F.D.A. approval of the hair ointment later this year, beginning a licensing process that usually takes about two years.
Cost Is $100 a Month
But thousands of men apparently have sidestepped the barriers to using this drug, with the help of medical doctors.
According to Dr. Reed, to convert minoxidil, the hypertension drug, into minoxidil, the hair treatment, 180 tablets are crushed and mixed with water, alcohol and propylene glycol.
That produces two ounces of the hair growth formula, enough for a one-month supply. Rob Davis, a colleague of Mr. Nordmann at Oppenheimer and one of Dr. Reed’s patients, said the formula was applied nightly from an eyedropper, rubbed in and covered with a cap. The cost is $100 a month, he said.
”I think it’s worked fairly dramatically,” said Mr. Davis, who said his existing hair seems to have thickened, although new hair growth has been less dramatic. Dr. Reed agreed that minoxidil held more promise for making existing hair look fuller, rather than for causing new hair to sprout.
According to a spokesman, Upjohn has been warning users that the ground-up tablets might produce different results from the ointment given to the volunteers in the Upjohn study. The F.D.A. agrees, said Bruce Brown, an agency spokesman, noting that the altered product ”may not bear any resemblance to the form that is under investigation.”
In its tests, Upjohn said, it has found that one-third of the volunteers showed hair growth, while on another one-third there was light, fuzzlike growth. The remaining volunteers were not helped by the ointment.
Its a part of your routine and you’re less likely to forget it, you’ll always be near it when you get out of bed, etc. But for night it can also be a part of your nightly routine and during your sleep you won’t have time to hyper focus on whether youre feeling sides or not, but I can also imagine that with nothing to stimulate your mind you may wander to whether you’re feeling sides while if you take it in the day you’ll have stuff to occupy yourself so you maybe won’t think about it.
Makes no difference when you take it because it is not the blood level that works, but the tissue buildup in the hair follicles.
The person who put these grafts into your recipient area seems to have only put the bottom of the graft into the recipient wound. I see most of each graft actually not inside the skin where they belong. I see quite a few grafts that seem to be already out as I zoomed into the photo. If this is what the ‘operator’ thinks is a quality hair transplant, they better go back to school and I am not sure how to help you at this late stage. I would doubt that most of these grafts will survive. There appears to be a great deal of work done and a good amount of your donor area has been harvested which will impact your next experience significantly.
[click to enlarge]
Dr. Rassman’s Comment: The study is small (15 patients) but the conclusion is significant that microneedling doesn’t help either grow hair or improve existing hairs. I don’t necessarily agree with the message delivered by these authors as we have been seeing considerable hair growth in many young men using microneedlin; nevertheless, I always strive to give both sides of the story.
SOHAG MEDICAL JOURNAL Vol. 22 No.1 Jan 2018 369 Microneedling as a monotherapy in treatment of male androgenetic alopecia Essam A. Nada, RehamEzz El-Dawla, Wafaa M. Abd El-Maged Ashraf A. Abd El-Latif, Marwa A. Abo Elmagd, Department of Dermatology, Venereology and Andrology Introduction Androgenetic alopecia (AGA) is the result of progressive, patterned hair loss that occurs when genetically predisposed individuals are exposed to androgens.The psychosocial impact of AGA may negatively affect patient’s quality of life andcan lead to personal social and job-related problems (1) .
Also; AGA can cause indirect physical harm to some patients, such as sunburn as a result of hair loss and exposure to ultraviolet light (2) .Moreover; AGA is reportedly associated with increased incidence of myocardial infarction, hypertension and hypercholesterolemia (3) . Drug therapies for AGA approved by the FDA are limited to topical minoxidil and oral FIN with efficacy varies between 40% and 60% (4) . Multiple factors are implicated in the pathogenesis of AGA which involves not only DHT but also inflammation, genes, signalling pathway, stimulatory pathways like Wnt/B catenin, and growth factors(5) . The existing conventional therapies (i.e. FIN and minoxidil) fail to target all of them (6) .
Microneedling (MN) is a relatively new minimally invasive procedure involving controlled puncturing of the skin by rolling with miniature fine needles(7) . It showed efficacy in some dermatological conditions including post-acne scars (8) , other scars (9) , pigmentary disorders (10) , and as a method of drug delivery (11) . The demand for new treatment techniques for AGA is growing, various procedures like mesotherapy, MN, platelet rich plasma, low laser light therapy, and stem?celltherapy are under active investigation (12) . MN creates multiple microchannels and increases transdermal penetration of drugs, facilitating higher concentration in dermis (13) . Scalp needling also stimulates blood flow around blood starved hair follicles and gently exfoliates dead skin cells (14) .ScalpMN also induces hair regrowth by the following: release of growth factors through platelet activation and skin wound regeneration mechanism, activation of hair follicle stem cells in the hair bulge area under wound healing conditions which is caused by MN, and overexpression of hair growth-related genes, vascular endothelial growth factors, ? catenin, Wnt3a, and Wnt10 b as documented in animal studies(6) . The use of MN in combination with minoxidil showed promising results in treatment of AGA (6) . Furthermore; the addition of MN to minoxidil and oral FIN improved AGA in patients who were resistant to minoxidil and oral FIN (15) . To the best of our knowledge; the use of MN as monotherapy hasn`t been previously reported.This study was designed to evaluate the efficacy and safety ofMN as a monotherapy in treatment of male AGA.
Patients and methods: After approval of this study by Ethical and Research committees at Faculty of Medicine, Sohag University;15 male patients complaining of progressive hair loss diagnosed as AGA were included.All patients assigned informed written consent. Exclusion criteria Exclusion criteria included patients with other forms of alopecia including telogen effluvium, alopecia areata, those with dermatological or systemic illness known to cause diffuse hair loss (as thyroid disorders or anemia), and patients received minoxidil, any hair growth promoters in the past six months or those underwent surgical hair transplantation. SOHAG MEDICAL JOURNAL Microneedling as a monotherapy in treatment Vol. 22 No.1 Jan 2018 Marwa A. Abo Elmagd 370 Patients on hormonal, androgenic or anti androgenic medications cytotoxic, and inhibitors of CYP3A4 (ketoconazole, verapamil, diltiazem, cimetidine, ciprofloxacin), and those on anticoagulant medications (aspirin, warfarin and heparin) or have skin disease with Koebner’s phenomenon were also excluded.
Methods:All patients were subjected to: I- Initial evaluation Personal history was reported including the age, occupation, marital status, smoking, and residency. History of hair loss was discussed with all patients including onset, course and duration of hair loss, site of hair loss, and use of hair care cosmetics (dying, bleaching, and straightening).History of other skin diseases, systemic diseases or any medications was documented. Family history of a similar condition was reported. Full general examinationwas done searching for any signs of anemia. Scalp examination was performed as regards any signs of inflammation, scales, erythema or scarring. II- Evaluation of hair loss: II.1- Pull test:After instructing the patient not to wash hair for 24 hours; grasping a small clump about 60 hairs in the index, middle and thumb was done, with pulling hairs gently but with firm pressure. The shed hairs were counted and positive test was reported as more than 6 hairs shed (16) . II.2- Grading of AGAaccording to Norwood-Hamilton scale(17) . II.
3- Digital photography: The patients were photo documented before and after six months of treatment. A picture of the frontoparietal region was obtained in each patient. Before the photograph was taken the patient’s hair was combed in a consistent manner for each patient so that the balding area could be optimally viewed. II.4- Trichoscopy: An epiluminescene digital microscope (compareview A/V; Version 1.5.09) was used in this study. Trichoscope has five magnification lenses of different magnification powers which are x15, x30, x 50, x150 and x200 folds.A magnification of (x 50) was used to detect the density of the hair follicles, vellus and terminal hairs; while magnification of (x 200) was used to evaluate the caliber of the hair shaft. The frontal area of the scalp of each patient underwent tricoscopic evaluation, before and six months after treatment (frontal area was defined as approximately 2 cm from the frontal hairline and 2 cm from the midline(18) . Trichoscopic images were taken at the same specific area in the frontal area of each patient. III- Treatment procedues:Microneedling was done using dermapen. Dermapen is auto-stamp motorized meso machine; it is a pen like instrument with a handle, a disposable needle cartridge, having 12 needles arranged in rows, and a power button to turn the machine on and off. It has guides to adjust needle length (turning the guide clockwise, the needle length will be longer ranging from (0.25 mm to 2 mm maximum). Topical anesthesia with 4% lidocaine cream was carried out 30 minutes before the procedure to the frontal area of the scalp of each patient, after this period, the cream was removed with saline, and the antisepsis of the entire area to be treated was subsequently performed with alcoholic chlorhexidine. Then the area had been completely dried. The affected area of the scalp was stretched and MN was carried out in vertical and horizontal directions for about four to five times, until mild erythema was noted. The used needle length was 1.25 mm.
Treatment schedule: 13 sessions were done for each patient according to the following schedule: ? Once every week for eight weeks (week 0, 1, 2, 3, 4, 5, 6, 7). ? Once every two weeks for one month (week 9, 11). ? Once every month for three months (week 15, 19, 23). IV- Treatment assessment: Assessment was done monthly and one week after the last session (24th week) depending on: IV.1) Grading scale: according to NorwoodHamilton grading scale. SOHAG MEDICAL JOURNAL Microneedling as a monotherapy in treatment Vol. 22 No.1 Jan 2018 Marwa A. Abo Elmagd 371 IV.2) Photographic assessment: Digital photos of the affected region were obtained from patients before starting treatment (baseline) and at the end of treatment . IV.3) Phototrichoscope: All patients underwent trichoscopic evaluation. Hair images were taken at 50x and 200x fold magnifications at the same affected area in the scalp of each patient before and after six months of treatment with (measurements of the hair density, hair thickness and terminal/vellus hair ratio(. IV.4)
Patient’s self-assessment (Hair growth questionnaire):Patients assessed their scalp hair at the end of the study using a validated hair growth questionnaire containing four questions on treatment efficacy and three questions on satisfaction with appearance (19) (Figure I). Figure I: Patient self-assessment questionnaire concerning changes in their scalp hair after treatment (19) . IV.5) Investigator assessment: The hair density in the fronto-parietal region was compared to that observed before treatment using a 7-point rating scale: greatly decreased (-3), moderately decreased (-2), slightly decreased (-1), no change (0), slightly increased (+1), moderately increased (+2), and greatly increased (+3)(20) . IV.6) Safety and tolerability assessment: Any side effects reported by the patient or noticed by the investigator was recorded. Statistical analysis: ? Data was analyzed using STATA intercooled version 12.1. ? Numerical data were presented as mean and standard deviation (SD) values, for parametric numerical data, while Median and range for non-parametric numerical data. ? For parametric data: Paired t-test was used to assess the statistical significance of the difference between two means measured twice. ? For non-parametric data: Wilcoxon signed rank test was used assess the SOHAG MEDICAL JOURNAL Microneedling as a monotherapy in treatment Vol. 22 No.1 Jan 2018 Marwa A. Abo Elmagd 372 statistical significance of the difference of non-parametric variables measured twice Results
The study included 15 male patients diagnosed as having AGA.The mean age± SD of the patients was 25.73±4.27 years, with 10 (66.67%) of them from rural areas.Family history of AGA was positive in 8 (53.33%) of the patients.Most of the patients 13 (86.67%) showed gradual onset of hair loss and progressive course was reported in all patients with mean disease duration ± SDwas 4.33±2.23 years.The highest prevalence of hair loss was in the frontal and vertex regions (53.33%).Only 2 patients (13.33%) had positive pull test and scales were found in 3 patients (20%). There was small decline in hair density but with no significant difference between hair density at the baseline and at 6th month.There was no significant difference between hair caliber at the baseline and at 6th month.There was mild increase in terminal/vellus ratio, with no significant difference between terminal/vellus ratio at the baseline and at 6th month (Table 1). Table 1: Results of phototrichoscopeof the study population (n= 15) before and after treatment. Variables At baseline After treatment Change Percent of change *P value Hair density: Mean ± SD Median (range) 189.07±143.1 187.5 (6-467) 180±130.48 150 (12-410) [-9.07]±30.30 0.5 ([-87]-22) -4% 0.65 Hair Caliber: Mean ± SD Median (range) 0.020±0.016 0.018 (0.001-0.05) 0.020±0.013 0.015 (0.004-0.045) 0.0003±0.015 0.001 ([-0.027]-0.031) 3% 0.69 Terminal/vellushair ratio: Mean ± SD Median (range) 2.72±3.88 1.75 (0.14-15.4) 2.89±5.06 1.45 (0.13-20) 0.17±1.40 -0.005 ([-1.3]-4.6) 4% 0.73 * P value <0.05 was significant. As regards the investigator assessment; 13/15 (86.67%) of the patients showed no change in hair density, one patient (6.67%) showed slight increase, and the last patient (6.67%) showed slight decrease. Results of the patient self-assessment questionnaire are shown in table 2. The only side effect was pain, which was reported in 10(66.67%) of the patients.
Table 2: Results of the patient self-assessment questionnaire in the study groups (n=15). Variables Frequency (Percentage) Variables Frequency (Percentage) 1) Bald spot getting smaller: Agree No opinion either way Disagree Strongly disagree 0 1 (6.67%) 7 (46.67%) 7 (46.67%) 2) Appearance of hair: Somewhat better A little better Same A little worse 0 2 (13.33%) 10 (66.67%) 3 (20.00%) 3) Hair growth: Greatly increased Moderately increased Slightly increased No change Slightly decreased 0 0 6 (40.00%) 7 (46.67%) 2 (13.33%) 4) Slowing down hair loss: Very effective Somewhat effective Not very effective Not effective at all 0 2 (13.33%) 8 (53.33%) 5 (33.33%) 5) Satisfaction about hairline at the front of the head: Very satisfied Satisfied Neutral Dissatisfied Very dissatisfied 0 0 3 (20.00%) 2 (13.33%) 10 (66.67%) 6) Satisfaction about hair on the top: Very satisfied Satisfied Neutral Dissatisfied Very dissatisfied 0 0 0 10 (66.67%) 5 (33.33%) 7) Hair overall satisfaction: Very satisfied Satisfied Neutral Dissatisfied Very dissatisfied 0 0 0 10 (66.67%) 5 (33.33%) SOHAG MEDICAL JOURNAL Microneedling as a monotherapy in treatment Vol. 22 No.1 Jan 2018 Marwa A. Abo Elmagd 373
Discussion The presence of AGAhas its negative effects on the patient’s quality of life(1) .As multiple factors are implicated in the pathogenesis of AGA and the existing conventional therapies (i.e. FIN and minoxidil) fail to target all of them; the demand for new treatment techniques for AGA is growing.MN is one of these techniques that showed promising results in treatment of male AGA (6, 15) . In the current study; there was statistically insignificant decrease in hair density with mean percentage of change (-4%) after treatment. This decrease can be taken as the “normal” hair loss with AGA, so patients go bald despite therapy. On the contrary; increased hair count was previously reported with use of MN in male AGA(6) . Authors found that MN along with minoxidil-treated group was statistically superior to minoxidil alone-treated group in promoting hair growth in men. The mean change in hair count after 3 months was significantly greater for the MN group compared to the minoxidil group (91.4 versus 22.2 respectively)(6) . This may be related to the value of MN as drug delivery method rather than mechanical effect. Concerning hair shaft diameter in the present study; there was minimal statistically insignificant increase with mean percentage of change (3%).The increase in the hair shaft diametergoes with the alleged role of trauma of MN in improvement of AGA.These results were consistent with a previous report of mild statistically insignificant increase in mean hair shaft diameter in patients receiving mesotherapy with saline, this goes with the alleged role of trauma of mesotherapy injection in improvement of AGA(21) . Concerning terminal/ vellus ratio;there was 4% mean percentage of change, which was mild and not statistically significant.The minimal improvement noticed might be attributed to stimulation of hair growth by the trauma which increases the blood supply to the HF, but it was minimal and not clinically or statistically significant
References 1. Hunt N and Mchale S: The psychological impact of alopecia.BMJ. 2005; 331(7522): 951-3. 2. Gubelin Harcha W, Barboza Martinez J, Tsai TF, Katsuoka K, Kawashima M: A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia.J Am Acad Dermatol. 2014; 70(3): 489-98.e3. 3. Kim MW, Shin IS, Yoon HS, Cho S and Park HS: Lipid profile in patients with androgenetic alopecia: a meta-analysis.J Eur Acad Dermatol Venereol. 2017; 31(6): 942-51. 4. Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D and Kouskoukis K: Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period.J Cutan Aesthet Surg. 2014; 7(4): 213-9. 5. Leiros GJ, Attorresi AI and Balana ME: Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/beta-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia.Br J Dermatol. 2012; 166(5): 1035-42. 6. Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A: A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study.Int J Trichology. 2013; 5(1): 6-11. 7. Fertig RM and Gamret AC: Microneedling for the treatment of hair loss? 2017. 8. Ayatollahi A, Hosseini H, Shahdi M, Ahmadnasrollahi S, Nassirikashani M: Platelet-rich Plasma by Single Spin Process in Male Pattern Androgenetic Alopecia: Is it an Effective Treatment? Indian Dermatol Online J. 2017; 8(6): 460-464. 9. Costa IM and Costa MC: Microneedling for varicella scars in a dark-skinned teenager.Dermatol Surg. 2014; 40(3): 333-4. SOHAG MEDICAL JOURNAL Microneedling as a monotherapy in treatment Vol. 22 No.1 Jan 2018 Marwa A. Abo Elmagd 374 10. Budamakuntla L, Loganathan E, Suresh DH, Shanmugam S, Suryanarayan S: A Randomised, Open-label, Comparative Study of Tranexamic Acid Microinjections and Tranexamic Acid with Microneedling in Patients with Melasma.J Cutan Aesthet Surg. 2013; 6(3): 139-43. 11. Iriarte C, Awosika O, Rengifo-Pardo M and Ehrlich A: Review of applications of microneedling in dermatology.Clin Cosmet Investig Dermatol. 2017; 10: 289-98. 12. Motofei IG, Rowland DL, Baconi DL, Tampa M, Sarbu MI: Androgenetic alopecia; drug safety and therapeutic strategies.Expert Opin Drug Saf. 2018. 13. Escobar-Chavez JJ, Bonilla-Martinez D, Villegas-Gonzalez MA, Molina-Trinidad E, Casas-Alancaster N: Microneedles: a valuable physical enhancer to increase transdermal drug delivery.J Clin Pharmacol. 2011; 51(7): 964-77. 14. Matarasso A, Pfeifer TM and Plastic Surgery Educational Foundation DC: Mesotherapy for body contouring.Plast Reconstr Surg. 2005; 115(5): 1420-4. 15. Dhurat R and Mathapati S: Response to Microneedling Treatment in Men with Androgenetic Alopecia Who Failed to Respond to Conventional Therapy.Indian J Dermatol. 2015; 60(3): 260-3. 16. Shapiro J, Wiseman M and Lui H: Practical management of hair loss.Can Fam Physician. 2000; 46: 1469-77. 17. Sehgal VN, Kak R, Aggarwal A, Srivastava G and Rajput P: Male pattern androgenetic alopecia in an Indian context: a perspective study.J Eur Acad Dermatol Venereol. 2007; 21(4): 473-9. 18. Hillmann K and Blume-Peytavi U: Diagnosis of hair disorders.Semin Cutan Med Surg. 2009; 28(1): 33-8. 19. Barber B, Kaufman K, Kozloff R, Girman C and Guess H: A hair growth questionnaire for use in the evaluation of therapeutic effects in men.J Dermatol Treat. 1998; 9(3): 181-186. 20. Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I: A randomized, placebocontrolled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss.J Am Acad Dermatol. 2004; 50(4): 541-53. 21. Sobhy N, Aly H, El Shafee A and El Deeb M: Evaluation of the effect of injection of dutasteride as mesotherapeutic tool in treatment of androgenetic alopecia in males.Our Dermatol Online. 2013; 4(1): 40-5.1. Muller DC, Giles GG, Sinclair R, Hopper JL, English DR: Agedependent associations between androgenetic alopecia and prostate cancer risk.Cancer Epidemiol Biomarkers Prev. 2013; 22(2): 209-15. 22. Hunt N and Mchale S: The psychological impact of alopecia.BMJ. 2005; 331(7522): 951-3. 23. Gubelin Harcha W, Barboza Martinez J, Tsai TF, Katsuoka K, Kawashima M: A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia.J Am Acad Dermatol. 2014; 70(3): 489- 498.e3. 24. Kim MW, Shin IS, Yoon HS, Cho S and Park HS: Lipid profile in patients with androgenetic alopecia: a meta-analysis.J Eur Acad Dermatol Venereol. 2017; 31(6): 942-951. 25. Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D and Kouskoukis K: Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period.J Cutan Aesthet Surg. 2014; 7(4): 213-9. 26. Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A: A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study.Int J Trichology. 2013; 5(1): 6-11. 27. Arif T, Dorjay K, Adil M and Sami M: Dutasteride in Androgenetic Alopecia: An Update.Curr Clin Pharmacol. 2017; 12(1): 31-35. 28. Abdallah M, El-Zawahry K and Besar H: Mesotherapy using dutasteride-containing solution in male pattern hair loss: A controlled pilot study.J Pan Arab Leag Dermatol. 2009; 20: 137-45. SOHAG MEDICAL JOURNAL Microneedling as a monotherapy in treatment Vol. 22 No.1 Jan 2018 Marwa A. Abo Elmagd 375 29. Sobhy N, Aly H, El Shafee A and El Deeb M: Evaluation of the effect of injection of dutasteride as mesotherapeutic tool in treatment of androgenetic alopecia in males.Our Dermatol Online. 2013; 4(1): 40-5. 30. Saceda-Corralo D, Rodrigues-Barata AR, Vano-Galvan S and Jaen-Olasolo P: Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia.Int J Trichology. 2017; 9(3): 143-145. 31. Fertig RM and Gamret AC: Microneedling for the treatment of hair loss? 2017. 32. Dhurat R and Mathapati S: Response to Microneedling Treatment in Men with Androgenetic Alopecia Who Failed to Respond to Conventional Therapy.Indian J Dermatol. 2015; 60(3): 260-3. 33. Shapiro J, Wiseman M and Lui H: Practical management of hair loss.Can Fam Physician. 2000; 46: 1469-77. 34. Sehgal VN, Kak R, Aggarwal A, Srivastava G and Rajput P: Male pattern androgenetic alopecia in an Indian context: a perspective study.J Eur Acad Dermatol Venereol. 2007; 21(4): 473-9. 35. Barber B, Kaufman K, Kozloff R, Girman C and Guess H: A hair growth questionnaire for use in the evaluation of therapeutic effects in men.J Dermatol Treat. 1998; 9(3): 181-186. 36. Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I: A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss.J Am Acad Dermatol. 2004; 50(4): 541-53. 37. Mounsey AL and Reed SW: Diagnosing and treating hair loss.Am Fam Physician. 2009; 80(4): 356-62. 38. Andriole GL and Kirby R: Safety and tolerability of the dual 5alpha-reductase inhibitor dutasteride in the treatment of benign prostatic hyperplasia.Eur Urol. 2003; 44(1): 82-8. 39. Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY: Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, doubleblind, placebo-controlled, phase III study.J Am Acad Dermatol. 2010; 63(2): 252-8. 40. Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM: The effect of 5alphareductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men.J Clin Endocrinol Metab. 2007; 92(5): 1659-65. 41. Olsen EA: Female pattern hair loss.J Am AcadDermatol. 2001; 45(3 Suppl):s70-80 ?????? ?????? SOHAG MEDICAL JOURNAL Microneedling as a monotherapy in treatment Vol. 22 No.1 Jan 2018 Marwa A. Abo Elmagd 376
The age gap just seems pretty arbitrary. Not sure if I should wait or just go for it. Thanks!
The issue here if you are 25, is what you have done prior to this time. Have you tried the drug finasteride? What has happened to your hair loss on the drug? This is the type of information I need besides your family history. I don’t want you to wake up one day and hate me if you became very bald one year after I did your hair transplant. I want to know as much as you do.
Page 209 of 1240