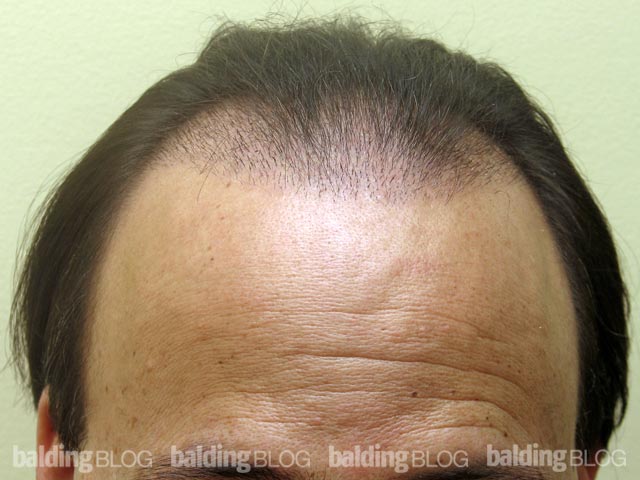
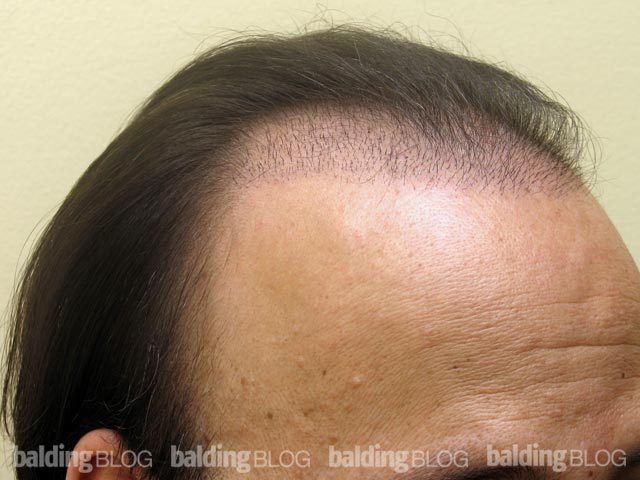
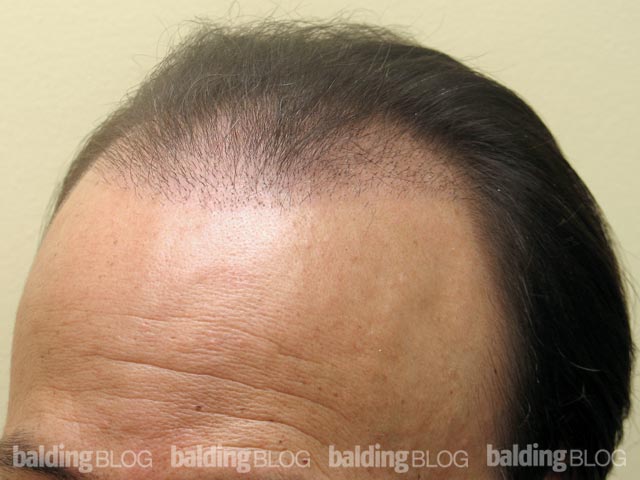
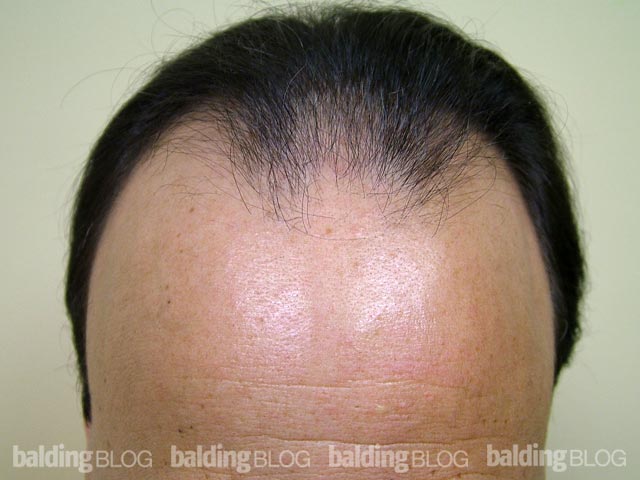
Greetings Dr. Rassman,
We’d like to thank you for taking the time to acknowledge the HairMax LaserComb on your Web site. We take this opportunity to respond to some of your comments and attempt to put to rest much of the ongoing debate over the HairMax LaserComb on your site.
In January of 2007, the HairMax LaserComb was Cleared by the FDA for the “Promotion of Hair Growth†in males with certain classes of Androgenetic Alopecia. This Clearance was based, not solely on a predicate device (as you’ve stated in your blog), but also on solid clinical data from our multi-centered double blind sham controlled study which followed all Good Clinical Practices.
For your readers reference, there are two processes for medical device approvals from the FDA, one being PMA (Pre-Market Approval) and the other is a 510K. Since our laser is considered a NSR (Non Significant Risk) device as defined by international laser safety standards, we appealed to the FDA to submit a 510K based on the safety of predicate devices.
Our clinical study, device labeling, GMP (Good Manufacturing Practices) and ISO (Quality) accreditation was the basis of receiving the FDA 510k Clearance.
The study took place at 5 sites throughout the United States. We are currently drafting a medical review of the study to be submitted to a peer-reviewed journal. We have every intention of publishing the study and making the full results available for public scrutiny.
Our statistical data, along with macro analyses of trial participants’ before and after images were proven to be medically significant. Our clinical protocol was IRB approved, followed GCP (Good Clinical Practices) and we utilized clinical trial monitors to verify all source data and case report forms. Please understand that we had to prove to the FDA, under the same statistical scrutiny as both Propecia and Minoxidil, that the HairMax was effective in increasing ‘Hair Counts’ in men with Androgenetic Alopecia.
In addition, we have just completed a clinical study for female Androgenetic Alopecia and have filed a new FDA 510K submission for females. We are cautiously optimistic that it will receive Clearance for females suffering from hair loss.
As for statements on your site concerning the difference between FDA approval and FDA clearance, here is the rule of thumb for submissions cleared by the FDA: In general, Drugs are ‘Approved’ for sale, and Medical Devices are ‘Cleared’ for an ‘Indication of Use’.
In your ongoing critique of the HairMax LaserComb, you also compare the technology to that of the laser hood. Here, again, you are not taking in to account the differences between the two devices. Yes, the HairMax LaserComb and the laser hood both use red lasers, but the HairMax uses a patented special array of hair parting teeth which allow for an unobstructed path of the laser to the scalp. We believe this direct laser path to the hair follicle is a critical design feature which makes the HairMax LaserComb standout from the laser hood and provides greater efficacy. Simple reasoning would indicate that an individual’s hair will block some of the laser energy from the laser hood and they would simply not see comparable results because the laser energy would not reach the scalp.
We have conducted an experiment aimed at assessing the delivery of our laser to the scalp with a specific emphasis on the efficacy of the HairMax LaserComb’s patented hair parting teeth. The results showed that 92% of the laser energy emitted from the HairMax is delivered precisely and accurately due simply to the movement of the hair away from the scalp by the hair parting teeth. The efficacy of laser therapy reaching the scalp of non-parted hair amounted to only 9-24%. Obviously this design feature is only valid for someone with hair.
Finally, your critique of the HairMax always seems to point to an alleged lack of clinical data and a clear mechanism of action.
Similar to Rogaine, how the HairMax LaserComb works is debatable and evolving. It is our hypothesis that the HairMax LaserComb, in some way, stimulates the dermal papilla leading to increased cell proliferation of the hair matrix. We believe this process causes increased production of the hair fiber. Supporting this hypothesis are user reports of faster growing hair.
We further hypothesize that the HairMax is an anagen inductor, and support this hypothesis by user reports of increased shedding at the onset of treatment. To us, this indicates an increase in telogen fallout leading to healthier anagen growth.
A few of your prominent colleagues suggest that the HairMax LaserComb may also have some effect on cell apoptosis, but we will not comment further as we know it will spark debate and we do not have any scientific premise to support this theory, yet.
We are about to conduct further research with histologic biopsies to document some of the changes which occur after HairMax LaserComb usage.
Please understand that when we went public with the fact that we were proceeding with clinical trials, naysayers complained that it was just a farce for us to attempt to increase business. Now that we have proven, through our FDA clearance, that clinical trials were conducted, some people still continue to be skeptics…The real issue seems to be that people are not willing to move away from the status quo. Rogaine and Propecia are approved; we are Cleared; all three of us have been proven as effective treatments for Androgenetic Alopecia.
We don’t guarantee that everyone will see results. From our extensive anecdotal experience spanning over 20 years, we have seen that 45% of users see benefits quickly, 45% of users see benefits over time, and 10% of people will see little results. Keep in mind, compliance is a major factor in the realization of benefits. In reality, treatments for any condition are never a sure bet, and we do not contend that our product is a miracle for hair loss sufferers. It does, however, offer well founded hope and quantifiable results, and that’s what we’re about.
To conclude, we have been collaborating with leading hair researchers and clinics across the world. What we’ve found is that the experts who actively use the HairMax LaserComb accept its efficacy; those who do not remain skeptical.
This seems to be the same issue we encounter with non-users of the HairMax LaserComb who are quick to complain that it is ineffective, but are unwilling to give it a chance to work. The HairMax LaserComb has been on the market since 2001, and one of the key factors in marketing a medical device is user experiences. We believe the HairMax LaserComb has one of the highest levels of customer satisfaction of any hair treatments. In addition to positive changes in hair growth, the quality, condition, tensile strength and manageability of the hair is enhanced. We offer a money back guarantee to our direct customers; if the HairMax LaserComb was not an efficacious treatment we would have been in the archives of a ‘hair loss snake oil’ schemes by now. Instead, we have achieved FDA accreditation and gained acceptance from hair experts and have satisfied users worldwide.
We hope that this clears up any misunderstandings you or your readers may have about our medical device.
Sincerely,
Lexington International LLC
I would love to believe that the technology works, but as yet, I can not become a supporter. I will look forward to your new publication and will read it with interest. I try to give controversial issues space on this blog to give a balanced view, so your response is appreciated.

 Full text of the press release
Full text of the press release  Dihydrotestosterone (DHT) is a male hormone, so I would expect that it might have some impact on muscle mass. Propecia blocks DHT and causes a rise in systemic testosterone by about 18%, so maybe there is an effect on muscle mass building stemming from an increased testosterone level, which is a much stronger male hormone than DHT. So indirectly, Propecia might be positive for muscle building if DHT levels go down (from the Propecia) and testosterone levels go up to compensate. Everyone is different, so I couldn’t say what is actually impacting muscle mass.
Dihydrotestosterone (DHT) is a male hormone, so I would expect that it might have some impact on muscle mass. Propecia blocks DHT and causes a rise in systemic testosterone by about 18%, so maybe there is an effect on muscle mass building stemming from an increased testosterone level, which is a much stronger male hormone than DHT. So indirectly, Propecia might be positive for muscle building if DHT levels go down (from the Propecia) and testosterone levels go up to compensate. Everyone is different, so I couldn’t say what is actually impacting muscle mass. If you have doubts about your doctor’s diagnosis or plan, maybe you need to see another doctor for a second opinion. Doing this over the Internet is virtually impossible. The flakes you get out should be examined microscopically. If you just have seborrhea, then there are a wide variety of over-the-counter shampoos for it. If it is psoriasis as your doctor has suggested, what is the plan in confirming the diagnosis and treating the condition? That is the role of the doctor taking care of you. If it were lice, your doctor surely would’ve noticed that.
If you have doubts about your doctor’s diagnosis or plan, maybe you need to see another doctor for a second opinion. Doing this over the Internet is virtually impossible. The flakes you get out should be examined microscopically. If you just have seborrhea, then there are a wide variety of over-the-counter shampoos for it. If it is psoriasis as your doctor has suggested, what is the plan in confirming the diagnosis and treating the condition? That is the role of the doctor taking care of you. If it were lice, your doctor surely would’ve noticed that.